Prosigna
Riesgo de Recurrencia del Cáncer de Mama
¿Por qué realizar este examen?
En los últimos años, la comprensión del cáncer de mama ha evolucionado significativamente, considerándose ahora una patología compleja y heterogénea. Por lo tanto, se deben considerar factores distintos para determinar el pronóstico del paciente. Las mujeres con cáncer de mama en etapa inicial que expresan receptores de estrógeno suelen tener un buen pronóstico. Sin embargo, en esta población, alrededor del 50% de las recurrencias ocurren después de cinco años del diagnóstico. Una necesidad inminente en el manejo del cáncer de mama positivo para receptores de estrógeno es identificar a las mujeres con un mayor riesgo de recurrencia tardía.
¿Qué es el examen?
El examen Prosigna (PAM50) se desarrolló a partir de una firma genética PAM50 que proporciona información sobre el riesgo de recurrencia del paciente (ROR – Risk Of Recurrence) basada en tres factores: tamaño del tumor, subtipo molecular intrínseco y estado de proliferación del tumor.
¿Para quién está indicado?
Mujeres posmenopáusicas que hayan sido mastectomizadas debido al cáncer de mama y que tengan las siguientes características:
- Cáncer de mama positivo para receptores hormonales, sin compromiso ganglionar, en estadios I y II;
- Cáncer de mama positivo para receptores hormonales, con compromiso ganglionar (1-3, 4 o más ganglios positivos), en estadios II o IIIA.
Tecnología
Sistema de Análisis nCounter Dx
Prosigna
Riesgo de Recurrencia del Cáncer de Mama
En los últimos años, la comprensión del cáncer de mama ha evolucionado significativamente, considerándose ahora una patología compleja y heterogénea. Por lo tanto, se deben considerar factores distintos para determinar el pronóstico del paciente. Las mujeres con cáncer de mama en etapa inicial que expresan receptores de estrógeno suelen tener un buen pronóstico. Sin embargo, en esta población, alrededor del 50% de las recurrencias ocurren después de cinco años del diagnóstico. Una necesidad inminente en el manejo del cáncer de mama positivo para receptores de estrógeno es identificar a las mujeres con un mayor riesgo de recurrencia tardía.
El examen Prosigna (PAM50) se desarrolló a partir de una firma genética PAM50 que proporciona información sobre el riesgo de recurrencia del paciente (ROR – Risk Of Recurrence) basada en tres factores: tamaño del tumor, subtipo molecular intrínseco y estado de proliferación del tumor.
Mujeres posmenopáusicas que hayan sido mastectomizadas debido al cáncer de mama y que tengan las siguientes características:
- Cáncer de mama positivo para receptores hormonales, sin compromiso ganglionar, en estadios I y II;
- Cáncer de mama positivo para receptores hormonales, con compromiso ganglionar (1-3, 4 o más ganglios positivos), en estadios II o IIIA.
Sistema de Análisis nCounter Dx
Ventajas
GRUPO SYNLAB
Garantizado por la experiencia del líder europeo absoluto en diagnóstico laboratorial.
COMPLETO
- Validado en dos estudios clínicos que evaluaron a más de 2,400 mujeres posmenopáusicas con cáncer de mama en estadio temprano.
- Aprobado por las agencias reguladoras de Estados Unidos (FDA) y Europa (EMA) como una herramienta pronóstica en el cáncer de mama inicial;
- Proporciona la evaluación del riesgo de recurrencia hasta por 10 años y el subtipo intrínseco.
Información Adicional
DOCUMENTACIÓN – Disponible en SYNLAB Direct para clientes
- Consentimiento Informado;
- Cuestionario Clínico;
- Solicitud Medica.
PREPARACIÓN
No es necesario estar en ayunas para realizar el examen.
Información Adicional
El informe incluye diferentes parámetros:
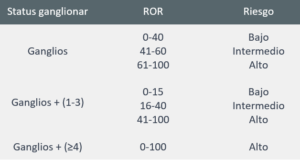
- Compromiso ganglionar y Subtipo intrínseco – clasificación de riesgo e información para las pautas terapéuticas.
- ROR: valor numérico de 0 a 100 relacionado con el riesgo de recurrencia en 10 años;
- Clasificación del riesgo: establece el riesgo de recurrencia en 10 años, clasificando en tres grupos posibles: Riesgo bajo, riesgo intermedio y riesgo alto.

Tiempo de Entrega
9 días laborables

Tipo de Muestra
Fragmento de tejido tumoral en bloque de parafina (consultar instrucciones de preparación del material).